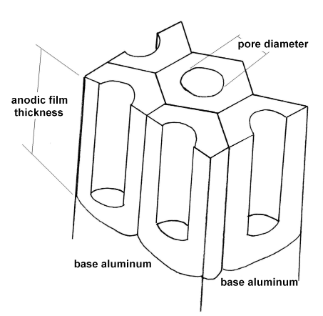
In nature, raw aluminum reacts with air and forms a very thin oxide layer on the surface. Once the thin oxide layer forms, the underlying aluminum is insulated from the air and the oxidation does not propagate. Anodizing is the process of forming the same oxide layer on aluminum as nature does, except anodizing does a much better job than nature. Anodizing produces an aluminum oxide layer, also called the anodic film that is thick, hard, and colourable. The structure of the anodic film is a network of honeycombs cells.
By varying the operating condition of the anodizing process, the thickness, hardness, and pore diameter of the anodic film can be controlled to achieve different properties.